News Story
BIOE Capstone Projects Focus on Medical Devices, AI, and More
On Monday, May 10th, the Fischell Department of Bioengineering (BIOE) Senior Capstone class presented 23 novel concepts in a live event that mirrored the traditional in-person competition. Projects ranged from a technique to treat cervical cancer from the pre-cancer phase in low- and middle-income countries, to a computer-vision assessment of fall risks for older patients or post-stroke patients. BIOE Chair John Fisher announced award recipients at the conclusion of the virtual event.
A total of 114 BIOE students pitched their products to a panel of 12 judges, BIOE faculty, and industry representatives in real time using Gatherly’s digital event platform. This allowed students and attendees to navigate from one project to the next as they would in person. It also enabled the four finalist groups – those awarded 1st place, 2nd place, 3rd place, and the Translational Design award – to present their projects to everyone in attendance.
This year’s panel of judges featured:
-
The Bioengineering Graduate Student Society
-
BIOE faculty members Lan Ma and Jarred Callura
 Team 1: Urine Particle Analysis in Body Fluid Images Using Machine Learning
Team 1: Urine Particle Analysis in Body Fluid Images Using Machine Learning
Tara Basir, Fadhili Maina, Ananya Mathur, Dana Ronin, Colin Skeen
Advisors: Dr. Steven Jay (BIOE Faculty), Dr. Jiong Wu, Dr. Matthew N. Rhyner, Jeremy Pettinato (Beckman Coulter)
This project was sponsored by Beckman Coulter
Urinalysis is a popular diagnostic test used in clinics, allowing for the detection of disorders such as urinary tract infections, kidney disease, and metabolic and system diseases. The current Beckman Coulter urinalysis technology, the APR system, uses an algorithm to categorize urine particles into 12 different particle types. While the system is able to correctly classify particles, there is only an 85% accuracy rate. Approximately 15% of the time, the algorithm is unable to classify the particle, producing an inconclusive result. In order to classify these particles, lab technicians are hired to analyze these samples using manual microscopy. While the test itself is inexpensive, ranging from $30-$60, it costs about $65-$70K annually to hire a lab technician thus significantly increasing the money needed to implement such a simple test. While manual microscopy produces accurate results, the process is labor intensive and tedious for lab technicians, ultimately making it inefficient. Team 1’s goal was to improve upon the current particle classification methods by developing an updated algorithm to reduce the need for lab technicians. Using images provided by Beckman Coulter, Team 1 developed three Convolutional Neural Network (CNN) models to classify particles, aiming for 95%-97% accuracy rate. Team 1 aimed for this level of accuracy to meet the standards for FDA approval. After developing the three models, they conducted accuracy assessments in order to determine the best model for predictive analysis. After analyzing the data and conducting accuracy assessments, the team determined that although the three models produced very similar results, Team 1’s sequential model worked best for image analysis. Overall, the team’s sequential model was able to analyze particles with a 93% accuracy rate and a 64% validation accuracy rate.
 Team 2: Injection of Ethyl Cellulose-Ethanol to Prevent Cervical Cancer
Team 2: Injection of Ethyl Cellulose-Ethanol to Prevent Cervical Cancer
Katherine Eckart, David Garvey, Philip Marcum, Marisa Patsy, Danielle Restaino
Advisor: Dr. Jenna Mueller (BIOE)
FIRST PLACE
Cervical cancer is the fourth most common cancer among women worldwide, with over 85% of cervical cancer-related deaths occurring in low and middle-income countries (LMICs). The disproportionate burden of cervical cancer in LMICs is largely due to limited access to trained providers and the biomedical technologies needed to diagnose and treat cervical pre-cancer before it becomes cancer. Current therapies for cervical pre-cancer, including procedures like Loop Electrosurgical Excision Procedure, cryotherapy, and thermocoagulation, are expensive, require trained doctors to perform, and are ultimately inaccessible at the point of care. Ethyl cellulose-ethanol (ECE) ablation has recently emerged as a low-cost, portable, and effective alternative treatment for cervical pre-cancer. However, in order to deliver ECE and reliably cover precancerous lesions of the cervix, a handheld injector that can control the needle placement and injection of ECE through a typical speculum is needed. To meet this need, Team 2 proposes a low-cost, portable, handheld device to enable the use of ECE in LMICs while limiting room for user error. This device uses a rechargeable battery-powered injection system, a mechanical needle actuation system, and a dual needle design to decrease treatment time. Major prototyping results include the construction of a low-cost, fully operational prototype that meets all necessary operational requirements including flow rate, needle insertion rate and depth, volume of delivery, and needle orientation. Final device printing will be completed using acrylonitrile butadiene styrene (ABS) in order to ensure the sterilizability of the device, and future work will include pre-clinical and clinical testing to achieve regulatory approval. The development of this device is a major step in providing affordable, effective, and accessible treatment options for cervical precancer to women in LMICs with the ultimate goal of decreasing cervical cancer mortality.
Team 3: Assessment of Fall Risk: A cell-phone application to conduct at-home fall-reduction training based on monitoring data
Alyssa Arminan, Martha David, Gloria Kim, Owen Roy, Diandra Youta
Advisors: Dr. Li-Qun Zhang (BIOE and UMB), Dr. Jiaqi Gong (University of Alabama), Dr. Angela Jones (BIOE)
This project was sponsored by Medtronic
SECOND PLACE
Stroke victims often suffer from the inability to fully control their muscle movements. In fact, stroke is the leading cause of mobility impairment and prolonged disability in the United States. The issue is worsened since patients are limited in how they interact with their doctors, and when they do, the tests performed are often subjective and qualitative. Elderly patients and those living in inaccessible regions are especially disadvantaged by lower frequency of clinic visits, which further restricts timely therapeutic interventions. If, however, doctors could quantitatively monitor a patient's movement profiles remotely, they could gauge their rehabilitative progress and adapt their treatment plans as needed, in an informed manner. Unfortunately, there is currently no solution on the market that fully addresses this need. Failure to resolve this issue results in unnecessarily prolonged treatments, delayed recovery, increased costs, and lower quality of life. Thus, Team 3 has designed a mobile application to collect and analyze gait patterns from the convenience of the patient’s own home. Using a combination of wearable bluetooth sensors, patients can track their routine movements with the touch of a button, while Team 3’s program runs on the background of their personal Android device. The recordings are then saved locally and securely stored in the cloud. Patients can then choose to share data with their physician, who can in turn access quantified metrics relevant to the patient’s stability and propensity of falls - such as their step duration, regularity, and symmetry. Doctors can translate this information into more accurate and prompt adjustments to the patient’s plan of care. Hence, this solution empowers doctors and patients to make optimal healthcare choices and enables high quality of care to all patients regardless of age and access to reliable transportation.

Team 4: Neonatal Bag-Mask Ventilation Controlling for Tidal Volume
Cassidy Craig, Joseph Forbin, Kylie Giordano, Margaret Reese, Leah Rock
Advisors: Dr. Kevin Cleary (Children’s National), Dr. John Idso (Children’s National), Dr. John Fisher (BIOE)
At birth, neonates must learn to breathe on their own after a long period of lung inactivity during gestation. This comes with a learning curve which differs from neonate to neonate, as 4.2% of all live births require assistance of some sort before gaining the ability to independently breathe. The window for such a diagnosis is short and requires immediate attention, as failure to receive oxygen will result in death. Healthcare providers respond to the situation using a bag valve mask (BVM) resuscitation system to deliver the clinically found tidal volumes of 4-6mL of air per kilogram of neonate weight. The harder the compression of the bag, the greater amount of air delivered. This demanding constraint may introduce strenuous circumstances for healthcare providers, often resulting in too great of a tidal volume delivery known as hyperventilation. This problem is amplified by the current standard for BVM resuscitation, in which healthcare providers deliver air to patients based on a pressure system. Furthermore, this is done with the same devices and practices to serve a wide range of patient sizes. This is critical, as this practice fails to recognize the nuanced and specific needs across such a wide range. Although this is not a completely inaccurate model, it is especially unaccommodating for neonatal patients (ranging from 0.5 to 5kg). This arises because the neonatal response to pressure based resuscitation does not suit their fragile lungs, resulting in 250,000 deaths globally (according to CDC estimates). To accommodate for these specialized needs and better serve neonatal patients, Team 4 hypothesized that the development of a new model that controls for tidal volume, as opposed to pressure, will result in a safer and more accurate method of delivering tidal volumes that are specific to neonates. To achieve this, the team proposes a retrofittable device that controls for tidal volume by placing guide handles around the bag, limiting the max distance by which a resuscitation bag can be compressed, regardless of external circumstances. In doing so, the chance of hyperventilation caused by human error will be eliminated. Fabrication of the prototype demonstrated the ability to deliver the desired tidal volume of 15 mL (the target value for serving a 3kg neonate) at a precision of 87%. This level of precision is found to be adequate following the clinical recommendation of 4-6mL/kg. The proposed device, when fully developed, will offer an assisting guide to healthcare providers and ultimately reduce the overall risk of the resuscitation procedure, putting both the provider and the neonate patient at ease.

Team 5: An Educational SARS-CoV-2 Genome Browser
Jessica Boyer, Matthew Brandon, Gillian Kramer, Elena Mirkovic, Sanjori Mukherjee
Advisor: Jared Callurra (BIOE)
The COVID-19 pandemic has drawn increased attention to the persistence and dangers of misconceptions, miscommunication and misinformation. These are social issues that cost the unnecessary loss of many lives over the past year, and continue to do so across the globe. This project was pursued in response to a need for strategies to more effectively communicate relevant scientific knowledge and research to the public. The approach this project took was to create a fully-functional, easily navigated, Educational SARS-CoV-2 Genome Browser within an educational SARS-CoV-2 resource website. The intended audience of the browser are high-school and college-aged students within biology-related course settings, potentially introduced by educators as a teaching tool. Through this avenue and other sharing platforms (news media, social media, internet searches, etc.), it is expected that the project will be able to gain attention and serve as a resource among interested members of the general public as well. The browser was created using the GBrowse browser creation platform, hosted on an Amazon Web Services (AWS) EC2 instance. The uploaded SARS-CoV-2 genomic reference sequence was retrieved from NCBI. The browser features key tracks including proteins of interest, variant mutations and restriction sites. The resource website was generated using WordPress, hosted on an AWS Lightsail instance. It features web pages explaining the biology of SARS-CoV-2, closely linked to the tracks displayed on the browser, alongside relevant public health information on COVID-19 such as vaccination and mask usage. A glossary and educational videos within the resource website further encourage users to learn new biology topics. Navigation between the browser and the resource website are facilitated through pop-up balloons on the browser and hyperlinks within the website. The prototype was launched for testing and feedback has been collected from over 40 students and educators. User experience within the site is being tracked by Google Analytics, which monitors the site’s user traffic. This data will be used to further improve the functionality of the browser and resource website. Certainly the project is untraditional with respect to past years’ Capstone projects, but a coding-based project allowed the team to adapt to the constraints of a virtual learning environment, successfully delivering a prototype that has already been implemented within course settings.

Team 6: Monitoring SpO2 at a site close to the injury of larger limbs
Tara Cecil, Katherine Dapkus, Michael Ryan McCreary, Juliana Pitzer, Gabriella Shahine
Advisors: Dr. William Bentley (BIOE, Fischell Institute), Dr. Li-Qun Zhang, (BIOE)
While current pulse oximeters perform well, they are limited in which areas of the body they can fit to and measure through. They can currently only detect SpO2 levels on small limbs like the fingertips and earlobes, with recent advancements allowing wrist measurements as well. Readings from these areas of the body provide general information about SpO2 levels, but are unable to provide localized SpO2 readings from large limbs like the arms and legs. Team 6 has designed a device for measuring SpO2 in these larger limbs using reflective pulse oximetry, in which red and infrared light are projected through the skin, reflected off of the hemoglobin present in the arteries beneath the skin,and measured by photodiodes present on Team 6’s device. The measurement of reflected light is then used by the associated device software to calculate the oxygen saturation of that specific region of the body. Additionally, the device is used with an adjustable band that allows it to fit around many body sizes. The cost of producing this device (less than $600) also makes it affordable for use in clinics and hospitals, including those in lower socioeconomic areas. These improvements to SpO2 technology will be useful for early detection of musculoskeletal injury or conditions that prevent proper oxygen distribution throughout the body. One such disease, called compartment syndrome, is common in military personnel and athletes due to their intense physical training. Use of this device could allow for early detection of associated reduced blood flow such that patients may avoid long-term effects of the disease, including needing amputation of the affected limb.

Team 7: Engineering β-cyclodextrin-based Nanoparticles for Sustained Release of Anti-Leukemia Therapeutics
Arjun Cherupalla, Samhita Chundury, Justin Morgan Longest, Nahom Michael, Praneeth Thota
Advisors: Tao Lowe (BIOE and UMB), Dr. Brian Blair (BIOE)
Additional information available upon request.

Team 8: Describing Inaccuracies in Wearable Heart Rate Monitors: A Dynamics Optics Simulation
Chenchen Handler, Nima Karodeh, Ann Rizkallah, Rebecca Vaudreuil, Ashley Williams
Advisor: Dr. Ian White (BIOE)
THIRD PLACE
The overarching goal of Team 8’s project was to investigate novel approaches to universally
improving the accuracy of wearable heart rate measurement devices via modeling and data
analysis techniques. After a thorough review of the literature, Team 8 chose to investigate five
possible sources of error in such measurements, including fat content, hair follicle density,
dermal thickness, skin tone, and the presence of sweat. The current literature has indicated that
these sources encompass the leading causes of inaccurate heart rate measurements in these
devices; however, only minimal in-depth research exists on any one of these. The purpose of
Team 8’s model is to correct for this downfall, leading to improvement in heart rate detection and, thus, minimizing the current accessibility barrier seen due to these physical attributes. Team 8’s design plan consisted of using the softwares SolidWorks and TracePro to create a tissue and capillary blood flow model, respectively. 40 skin models with varying optical properties were generated and tested through this model. Python was then used as a means of data processing and quantifying error, as it could directly take input from SolidWorks and TracePro in order to assess the accuracy of Team 8’s model. It was found that 1) dermal fat, superficial sweat, and melanin all decrease the flux of photons and reduce ray intensity as rays arrive at photodetectors, 2) hair follicles decrease the total flux to photodetectors by misdirecting rays, and 3) dermal thickness has no effect on readings. Due to the nature of this project and the resources currently accessible, Team 8’s budget was solely dependent on the cost of licensure for the TracePro software. Team 8’s next step is to deploy Team 8’s model into the fields of optics and sensors by consulting large corporations that mass produce inaccurate devices.

Team 9: Telerehabilitation platform to analyze 3D movement of stroke patients performing occupational tasks
Jennifer Biaksangi, Shawn Byrne, Chloe Keller, Catherine Levi, Fatima Mikdashi
Advisors: Dr. Kim Stroka (BIOE), Dr. Richard Macko, Dr. Charlene Macko (UMB), Dr. Giovanni Vincenti (University of Baltimore)
MPOWER AWARD
Stroke is the third leading cause of acquired adult disability worldwide, and its incidence continues to increase with the aging population. Even after months of rehabilitation, many stroke survivors suffer from a range of motor impairments. While traditional in-person rehabilitation has proven successful in regaining motor function, the approach presents challenges ranging from transportation to long-term insurance coverage. Moreover, in the wake of the COVID-19 pandemic, stroke survivors-- most of whom fall into at-risk age groups-- may feel hesitant to resume in-person treatment due to health concerns. As an alternative, the physical therapist (PT) might prescribe a set of exercises for the patient to do on their own
at home; however, most studies report less than 50% adherence to such treatment, largely due to a lack of guidance and patient fidelity to the routine. Depth-aware Automated Rehabilitation (DARe) addresses these obstacles by providing the patient with a virtual platform for interactive, personalized rehabilitation from the convenience of their own home. This novel approach couples LiDAR technology, used to capture patient movement in 3D, and artificial intelligence, which tracks 18 anatomical landmarks throughout the movement, without the need for physical markers and sensors. The accuracy of this approach was compared against OpenPose, the industry standard for markerless human pose estimation (but which relies on multiple calibrated RGB cameras to track 3D movement, as opposed to the single LiDAR camera needed for DARe), and returned an error of < 1.0%. The motion capture and analysis software has been prototyped in Python and patient/therapist interfaces have been refined through user experience testing. Within the platform, the PT can communicate with and prescribe custom rehab routines and goals to their patient. The patient can then use the LiDAR camera to record their sessions at home and receive immediate performance-based feedback in their profile. At the same time, a more quantitative report is sent to the PT for record-keeping and analysis. As LiDAR sensors are quickly becoming standard in mobile phones (e.g. Apple’s iPhone 12 Pro) and tablets due to the increasing popularity of augmented reality, the DARe platform will eventually take the form of a mobile application that can operate on the built-in camera; however, the video processing/analysis software and the clickable prototype of the interface have not yet been integrated. As health care models shift from service-based to results-based reimbursement, DARe’s quantitative approach to rehabilitation could be covered by insurance for long-term recovery, enabling stroke survivors to continue regaining mobility and independence past the acute recovery period. Moreover, DARe will provide increased availability to structured, easily accessible physical therapy for vulnerable populations while bringing stroke rehabilitation into the telehealth realm. This may help level the playing field so that underserved and under-insured persons of need can receive better, more accessible care.
Team 10: 3D-Bioprinting Biomimetic Materials to Differentiate Stem Cells into Osteoblasts for Bone Regeneration
Demitra Karalis, Priscilla Lee, Emory Charles Mummert, Caroline Olson, Zoe Roussos
Advisors: Dr. Tao Lowe (BIOE, UMB), Dr. Katharina Maisel (BIOE)
In the United States alone, millions of people each year suffer from bone density issues; over 50% of men and women over the age of 50 are living with some sort of bone density issue. Bone tissue has very limited regenerative ability and will not regrow defective bone on its own. Therefore, a therapy that allows for enhanced regeneration of this bone tissue is required. Our solution is to begin developing a 3D printed bioengineered scaffold constructed with collagen and hyaluronic acid that will allow for dental pulp stem cell differentiation and proliferation. This device and the cells would then be implanted into a deficient area of tissue, helping to regenerate that area of tissue. This could be used to treat osteoporosis by implanting the scaffold into the area of low-density bone or placing the scaffold at the center of a complex fracture. Once the cells begin proliferating and differentiating, new bone tissue could be grown to replenish the diseased and deficient areas. The majority of our prototyping included troubleshooting the 3D bioprinter and designing prints that were feasible with our Allevi 3 bioprinter. Through literature search, the optimal pore size was deemed to be 100 micrometers and would allow for the best possible cell growth and adhesion. However, the printer was originally unable to print our original design with the desired accuracy. With too many of these pores, the cube prints solid with no pores and space for cells to grow. Many alterations were made to the original design by reducing the number of pores and removing the infill settings. Eventually, the team was able to successfully print a hollow cube with singular pore on one face of the cube with the test material pluronic-127. After many alterations to pH and extrusion needles, this scaffold shape was successfully printed with the lab prepared collagen. Our project has the potential of improving the lives of many people suffering from bone deficiencies and other disorders. By forming a therapy that uses allogenic stem cells, risks of implant rejection are greatly reduced, allowing for a much safer treatment of these bone deficiencies in comparison to current treatment methods. Since the scaffold can be repeatedly produced using a bioprinter, using readily attainable materials, our solution would heavily improve the treatment of bone disorders.
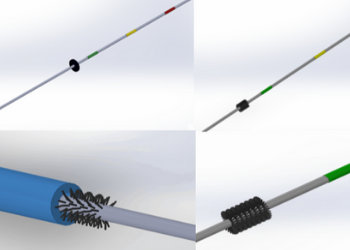
Team 11: Vascular Guidewire with Microvalve to Prevent Inadvertent Loss Within Body
Julia Cicalo, Cesar Funes, Sarah Levendusky, Celia Maiorano, Blake Michael Zucco
Advisors: Dr. Alisa Clyne (BIOE), Dr. Ron Samet (UMB)
Central line placements are integral for many surgical and medical procedures. During central line placement, there is a 1 in 3,000 chance that the guidewire used in this placement will get lost within the patient's vasculature. A lost guidewire is a critical and potentially life threatening complication in central line placements. The goal of this project was to solve the problem of lost guidewires in patients during catheter placement. Team 11’s solution was to integrate a stopping mechanism onto the guidewire that allowed the wire to pass through the central line catheter, but stop the advancement of the wire into the body if the patient were to suck the wire in through spontaneous breath, or the physician made a mistake and advanced the wire into the patient. Team 11’s prototyping resulted in a theoretical proposed design and a final prototype. The theoretical design uses manufacturing machinery to integrate a region of bristles into the guidewire that will flex to allow the catheter to pass, but still stop wire advancement into vasculature. The prototype design was a washer that would have to be added as an additional step to the guidewire after the wire has been threaded through the catheter. Modifying the current guidewire to have an additional safety mechanism would greatly reduce the chances of a wire getting lost in the vasculature and eliminate causing unnecessary injury to the patient.

Team 12: Automated Feature Detection for Custom Conformal Respirator Design
Deborah Acheampong, Zachary Dorsey, Jae Jung, Sojeong Lee, Trevor Mollot
Advisors: Kevin Aroom (Fischell Institute, BIOE), Dr. Giuliano Scarcelli (BIOE, Fischell Institute)
BEST VIDEO PITCH
The COVID-19 pandemic has introduced a new set of challenges to all individuals,
especially for healthcare workers who put themselves in harm’s way to care for their patients. While current N95 respirators have efficient filtration of airborne particles, they are not customizable, reusable, or transparent. Thus, current respirators do not provide perfect seal, cause environmental concerns, and prohibit good communication between individuals. Team 12’s project will significantly decrease the workflow by removing a labor-intensive step of positioning several components on top of 3D scans of individuals’ faces and produce conformal respirators with customized fit for individuals, transparent, reusable and more affordable. The process begins with a scan of the user’s face, using an Artec Leo scanner. The face model is then imported into Meshmixer and with the use of a Python script, the model is correctly oriented along the defined plane. The oriented mesh is exported from meshmixer and imported into the Autodesk Fusion 360 software. An automated Python script produces a thin flange body that represents the face contours that interface with the respirator. The resulting customized flange is printed using fused deposition modeling for each unique customer; the reusable mask solids themselves are only printed once with resin stereolithography. To form a complete thermoforming mold, the mask solid is slid through the flange in a predetermined orientation. Using this mold along with PET, a thermoforming process is used to generate the conformal respirator. The respirators can then be fitted with N95 filters and straps to secure them on the user’s face. This final product is tested using a Portacount fit tester 8048 to ensure a consistent seal. The production and use of the conformal mask poses little to no ethical concerns. Rather, the conformal mask has a positive benefit-risk relationship in that it decreases the emission and transmission of the viral particles. At large, this benefits the target population and brings us one step closer in defeating a virus that has taken the lives of many.
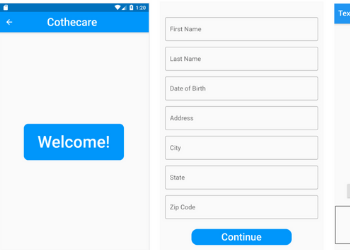
Team 13: COVID-19 App Suite for Contact-Free Patient Screening
Kraus, Samantha, Yutong Liang, Darshi Shah, Alana D. Tillery, Jillian Weiss
Advisors: Dr. Ian White (BIOE), Dr. Joseph Rabin (UMB)
As a result of the COVID-19 pandemic and the looming threat of viral exposure, many individuals in the United States are wary of gathering indoors and meeting others face-to-face, abiding by CDC guidelines to allow adequate social distancing. Unfortunately, even attending regular physician appointments and check-ups for pre-existing or nascent health issues poses an increased risk for contracting and spreading the virus. Therefore, the use of telehealth platforms has increased over the past few years and holds particular promise in this period for contact-free physician appointments and consulting. However, current issues with existing telehealth platforms include electrical transmission of private information, conducting and monitoring patients during specialized tests such as imaging and nasal swabbing, and increased chance of misdiagnosis without direct patient-physician interaction. Team 13’s pilot program, Cothecare, aims to resolve the current issues and downfalls of telehealth. Team 13’s mobile app suite, developed alongside guidance and approval from a physician and trauma center specialist, securely stores patient information and connects via private message or call to a physician with a working relationship established with the patient. The system allows for daily logging of coronavirus and asthma related symptoms and provides a diagnosis with suggestions for care based on these symptoms. Team 13 aims to improve outpatient care and increase access to health care professionals and physicians in order to diagnose and receive guidance on COVID-19 and asthma by developing a mobile app suite that acts as proof of concept for using telehealth as a method for treatment and patient connection for infectious diseases and chronic conditions.
Team 14: Radiomics Feature Prediction of Survival in Patients with High-Grade Gliomas
Michael Buckberg, Sabrina Cauton, Katherine Dura, Claire Rutkowski
Advisors: Dr. Lei Qin (Harvard Medical School), Dr. Yang Tao (BIOE)
Gliomas are tumors of the brain or spinal cord that are currently incurable, but are treatable
depending on the phenotype of the specific glioma. The phenotype of certain gliomas can be
determined by analysis of MRI scans. The objective of this project is to design a user-friendly,
interactive application that can objectively analyze specified extracted radiomics features from
MRIs of high-grade brain gliomas to predict patient survival for treatment evaluation. Team 14 has designed a random forest model built into an interactive app using Python. This model has been trained on publicly available glioma MRI images and it can classify an individual patient into short (15 months) overall survival time based on the patient’s own MRI. With this program, Team 14 hopes to assist physicians in developing a treatment plan for the high-grade glioma patients by aiding in their prognostic capabilities.
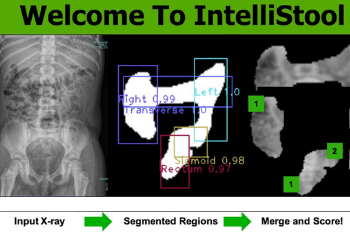
Team 15: Artificial Intelligence to Read Abdominal X-Rays as Part of a Bowel Management Program for Children with Constipation and Fecal Incontinence
Ali Aslam, Chaitali Chitnis, Jorge Guzman, Nealyn Ashraf Jahangir, Keerthana Srinivasan
Advisors: Dr. Silvina Matysiak (BIOE), Dr. Marc Levitt (Children’s National), Dr. Kevin Cleary (Children’s National)
Many children suffer from bowel complications such as constipation and fecal incontinence. These children are enrolled in a week-long bowel management program during which they undergo daily abdominal radiographs. Radiographs need to be analyzed by colorectal surgeons or radiologists to determine the appropriate treatment for the patient; however, many clinical facilities around the world do not have access to these experts. To address this problem, Team 15 developed IntelliStool, a software application that harnesses artificial intelligence to analyze the abdominal X-rays of patients enrolled in bowel management programs. Team 15’s process uses three different models of convoluted neural networks (CNN) to detect the colon, isolate its anatomical segments, and score their stool quantities. Team 15’s algorithm works by submitting the original image through a U-Net algorithm for image segmentation, which results in a mask that isolates the colon’s contour. Then, the image is analyzed by YOLO, an object detection algorithm, that improves specificity in the identification of the colon’s anatomical segments. Finally, a score prediction model analyzes the stool content of the individual segments on a scale from 0 to 2 using a CNN. Providers can upload X-ray images and receive scoring results through a graphical user interface (GUI). Ultimately, Team 15 was able to develop an algorithm capable of identifying the colon in an X-ray, isolating the colon segments, and then extracting them for stool quantity scoring. Team 15’s accuracies for the respective code segments were 65% for colon identification, 69% for segment extraction, and 53-70% for scoring depending on the colon segment being evaluated. A graphical user interface was also successfully implemented to walk users through the use of the software. One of the biggest ethical issues present in the field of medicine is the struggle for many individuals to have access to essential medical care. In fact, according to the U.S. Census Bureau, 27.5 million Americans had no access to health insurance in 2018. This problem is even more evident globally according to the World Health Organization, with more than 400 million people around the globe not having access to basic health care. Bowel Management Programs contribute to this ethical issue posed by medicine. Prior to the implementation of the treatment program, radiologists are needed for X-ray interpretation of patient colons. According to the Global Radiology Gap, round 67% of the world does not have access to radiology services, resulting in a large portion of individuals unable to have access to proper Bowel Management Programs. Intellistool tackles this ethical problem and as a result, access to proper Bowel Management Programs can become globalized to regions without radiologists.
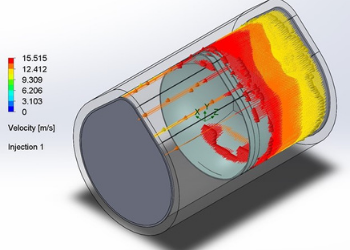
Team 16: Using Airflow Simulations to Design a More Efficient and Cost-Effective N95 Facemask
Mary Carbonell, Asma Farooqui, Diego Laboy-Morales, Christian Lazaro, Lina Tchangalova
Advisor: Dr. Lan Ma (BIOE)
Since the start of the global SARS-CoV-2 (COVID-19) pandemic, 477,789 healthcare professionals have contracted the virus with 1,565 of these individuals having died as a result, as of April 30, 2021. A major contributor to this rate of contraction is the still present shortage of N95 masks, resulting in nearly 50% of healthcare professionals reporting reuse for up to two
months, during which they are operating in highly contagious situations. These N95 masks are not designed for reuse beyond 4 to 5 uses due to the specific manufacturing methods that ensure filtration efficiency. This reuse also requires sterilization methods like bleach that damage mask integrity, contributing to the high rates of infection. An improvement of the N95 mask that allows for greater reusability with maintained filtration and structural durability is necessary to reduce the health risk to healthcare professionals, minimize environmental waste, and lower manufacturing costs. In response to this, Team 16 designed a fluid simulation model in SolidWorks that allows for the material selection of a particular mask design to be tested for filtration efficiency via varying particle sizes, concentrations, and flow velocities. A three-layered N95 mask model with an inner and outer layer composed of either nonwoven polypropylene, polyester or cotton and a melt-blown polypropylene middle layer was modeled using particle studies to simulate breathing, coughing, and sneezing situations. From this it was found all three materials passed the 95% filtration efficiency, but cotton was the most consistent and best fit the previous literature data, making it Team 16’s material of choice. The outer layer of this mask was made hydrophobic in order to filter liquid aerosols and for improved comfort and an enhanced fit, a silicon seal was added at the nose bridge, allowing for better sanitization, and structural durability. Physical prototypes of the N95 mask model were then fabricated and tested for filtration efficiency to determine durability after repeated moist heat sterilization in a microwave. This method was chosen based on literature studies that found moist heat was comparable to other approved sterilization methods such as autoclaving, and due to the easily
available device for at-home cleaning. Team 16’s results show that there is no statistically significant difference in filtration efficiency pre- and post-sterilization, making this an effective method for the team’s proposed mask design. The creation of the SolidWorks model will allow for an easy to use system by other researchers to test different mask designs and materials for simulated filtration efficiency. Team 16’s prototype testing shows that making a cotton mask that can be cleaned using moist heat sterilization is an effective alternative to current N95 models, which will allow for the safer reuse of the team’s mask by utilizing a more effective and easier at home cleaning method. This proposed mask construct will hopefully minimize both future infection rates and the environmental impact during this pandemic and any future health care crises.
Team 17: Custom Radiolucent Alignment Board For Intraoperative Lower Extremity Deformity Correction
Ryan Lee Everich, Anna Filatova, Shreya Khanna, Akorede Olayiwola, Vaani Shah
Advisors: Dr. Ed Eisenstein (BIOE), Dr. Megan Young (Children’s National)
Complex lower extremity deformities often require surgical reconstruction to restore normal alignment. Accurate measurements are needed to assess body alignment. However, there is currently a lack of proper tools to do so intraoperatively. There is currently no standard instrument to assist with intraoperative lower extremity deformity correction. The current methods are inefficient with a potential for inaccuracy and time consuming, with the risk of excess radiation exposure to the patient. The goal of this project is to create a custom board with an alignment grid specific to patients with a multiplanar deformity or multi-bone deformity
requiring a specific angle of correction. Surgical plans can include multiple correctional surgeries each with a target angle to obtain. This custom radiolucent alignment board will allow the surgical team to utilize any angle needed as a reference for alignment.
The objectives of this project include developing a radiolucent board with radiopaque
grid lines. Team 17’s final product consists of a 3D printed PLA board with lines engraved into the board. These lines house radiopaque copper wire of various thicknesses, which can be modularized to fit in angles from 83-90 degrees. Before converging upon this final design, Team 17 circulated through various prototypes and materials for Team 17’s board and grid lines. The team explored combinations of carbon fiber and plexiglass boards with ABS or barium sulfate grid lines, based on their material properties such as Z values. After developing a few prototypes testing grid line definition and time, the team decided to switch direction to PLA and copper due to its ease of use. This solution to a multifaceted problem will allow for less personnel working on the patient and allow simultaneous measurements to be taken at one time. The custom board will improve alignment accuracy and reduce operating times and radiation exposure for all Involved.
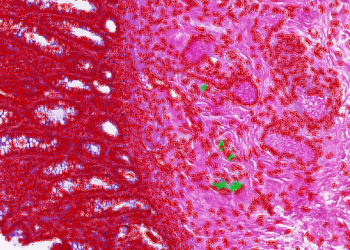
Team 18: Artificial intelligence for Reading of Hirschsprung's Pathology Slides
Lina Betu, Eyram Koudji, Cole Marra, Caitlyn Nguyen, Prateek Swamykumar
Advisors: Dr. Xiaoming (Shawn) He (BIOE), Dr. Marc A. Levitt (Children’s National)
BEST ABSTRACT
Hirschsprung disease is a congenital condition of intestine innervations present in 1 in
5,000 newborns. The disease results in a lack of ganglion cells in the area of the myenteric
(Auerbach) plexus and submucosal (Meissner) plexus in the distal section of the large intestine in an infant. The absence of the ganglion cells causes muscles in the bowel to lose their ability to move stool through the intestine as well as submucosal nerve hypertrophy. The main treatment is pediatric surgery to remove the affected bowel segment. Precise and quick diagnosis of the disease is the key to accurate treatment. The diagnosis is done mainly by biopsy of the affected bowel. From the biopsy, the complete absence of ganglion cells in the submucosal or intramuscular nerve plexus of the intestinal wall and the presence of hypertrophic nerve fibres and trunks has to be confirmed by a pathologist. There are some difficulties associated with the diagnosis and the proper recognition of ganglions cells, even with proper training. These issues are exacerbated in developing countries due to a lack of pathology technology and talent. In this project, QuPath and Python have been used to identify ganglion cells within patient rectal biopsy samples to aid in the diagnosis of Hirschsprung disease and improve pathological analysis at Children’s National Hospital. A random forest classifier was built in QuPath to correctly identify ganglion cells. Then, an automation script was coded in Groovy to streamline the process of detecting all cells, identifying ganglion cells with the classifier, and outputting cell measurements within QuPath. Using Python, Team 18 ran a correlation study to determine which features best separated ganglion cells from other cells. Team 18 built three additional classifiers in Python and compared them to their QuPath classifier. The QuPath classifier outperformed all three in sensitivity, but had a lower precision in detecting ganglion cells. The artificial intelligence program developed by the team’s project can be incorporated into an application that can be used worldwide to improve the quality of care of Hirschsprung disease globally. With further collection of samples an important database can be built while protecting patients data and rights and respecting their anonymity and confidentiality.
Team 19: Computer Vision Assessment of Fall Risks with Machine Learning for Older People or Patients Post-Stroke
Aodu Guo, Philip Kloner, Benjamin Lee, Emersen McCoy, Mark Melvin
Advisors: Dr. Helim Aranda (BIOE), Dr. Li-Qun Zhang (BIOE/UMB), Dr. Yang Tao (BIOE)
Falls remain the most common cause ofinjury among the geriatric population. These falls can cause numerous consequences to their health, as it causes bone perturbations, fractures, and breaks, leading to not only decreased quality of life post-injury, but also costly medical fees. There is a need to utilize preventative and rehabilitation programs to reduce risk of geriatric falls, but there is a lack of biomechanical research to support the development of such programs. With this, Team 19’s project aims to develop an artificial-intelligence tool that can predict a patient’s risk of anterior, posterior, medial, lateral, and collapse-directed falls through the analysis of joint movements. First, videos of mimicked falls were taken using a LiDAR L515 camera, an infrared camera with specific depth perception, and Cubemos SDK, a skeletal tracking software. The combination of the two allowed for the tracking of XYZ coordinates of each joint over time, which were used to train a Python-based neural network using a Sigmoidal weighted learning curve to output a 6 by 1 matrix predicting the likelihood of a fall mechanism in a patient at each frame. Using this produced dataset (n = 50 for each type of fall), the accuracy of this model was tested, which was determined by calculating the percent difference of the output to what was expected at each time frame. With the current dataset, a percent accuracy of 32% was achieved, although this should improve with more data. Team 19 offers a proof-of-concept tool that can be employed by physical therapists to develop training regiments and evaluate their effectiveness.

Team 20: Ultrasound Guided Pediatric Hip Aspiration Training Phantom
Shahed Bader, Christopher Garliss, Sandra Lavrenov, Brittney Murugesan, Devon Strozyk
Advisors: Dr. Catherine K. Kuo (BIOE), Dr. Evan Sheppard (Children’s National), Dr. Kevin Cleary (Children’s National)
ADVISORY BOARD AWARD FOR TRANSLATIONAL DESIGN
Pediatric septic hip arthritis is an infection in the synovial fluid and joint tissue of the hip in children. This is an uncommon infection in children which carries a poor prognosis if not properly diagnosed and treated in a timely fashion. Currently, the most accurate and specific diagnostic test for this infection is a needle aspiration. However, due to the rareness of this infection only a very limited number of physicians receive training in ultrasound guided needle aspiration. In order to make the practice of aspirating a suspected septic hip in the emergency room more widespread, more accessible, and increasingly accurate, training tools need to be developed. Team 20’s solution was to develop a high fidelity hip aspiration phantom that can endure multiple aspirations with a needle and has realistic ultrasound and anatomical landmarks. The team conducted extensive research to determine the biomaterials to use for each anatomical feature in the phantom. The femur and hemipelvis were 3D printed using PLA, ballistic medical gelatin was used to represent the muscle, an ambu bag was used to create the joint capsule and was filled with water-glycerol solution to mimic the synovial fluid, PTFE tubing and wires used to mimic the blood vessels and nerves, respectively, and agar gel was used to mimic the skin. All these components were assembled together using a 3D printed mold. Preliminary testing was conducted by medical professionals experienced in the procedure to determine the viability of the prototype. The phantom was evaluated based on a number of criteria under physiological accuracy and the realism under ultrasound. The results of the testing determined that the joint capsule was sufficiently reusable for up to 23 punctures without leak, the materials that represented the anatomical features were clearly visualized under ultrasound, and the model in general provided a real tactile feel of the procedure. Medical training phantoms are a powerful teaching tool with an important moral claim: to keep patients safe while training the next generation of clinicians and retraining current clinicians so that they are kept up-to-date. The use of this hip phantom in clinical settings would reduce reliance on the limited number of clinicians present that have training experience, extraction of fluid for diagnostic testing will be performed with increased accuracy, and training would be more widespread and provided for clinicians in various specialities.
Team 21: Building Supervised Machine Learning Models on Hematology Data to Aid in Diagnosis of COVID-19
Rohan Laljani, Rebecca Mathew, Justin Turner, Vinay Veluvolu
Advisors: Dr. Hubert Montas (BIOE), Glenda Holderbaum (Beckman Coulter), Dr. John Riley (Beckman Coulter), Dr. Carlos Ramires (Beckman Coulter)
This project was sponsored by Beckman Coulter
SARS-CoV-2, which presents in humans as COVID-19, has taken the world by storm for the past year. Testing has been a crucial tool in the fight against COVID-19, as asymptomatic individuals can still be contagious for up to two weeks. As more of the global population becomes vaccinated, testing may diminish, but accurate, affordable, and accessible diagnostic tests will still be important in light of global vaccine shortages, anti-vaxxer movements, possible infection of vaccinated persons, and new emerging strains of the virus. Previous research has shown the utility in designing diagnostic systems using machine learning models, as they have the ability to pick up on multiple pieces of patient information to produce an accurate diagnosis. The usage of supervised and/or unsupervised learning models has the ability to improve medical care and decrease cost. Considering the urgency of this pandemic, Beckman Coulter proposed the assessment of hematological parameters from their DxH 900 analyzer in order to develop a machine learning algorithm to aid in rapid and accurate diagnosis of COVID-19. As such, Team 21 developed a machine learning platform to diagnose COVID-19. The team’s test takes a 165 µL blood sample, processes it in 10 minutes, and provides a binary yes/no diagnosis with a 97.5% balanced accuracy, a 2.95% false positive rate, and a 16.07% false negative rate. These results are comparable to existing antigen and RT-PCR tests, and the rapidity and accessibility of Team 21’s test makes it a viable market alternative.
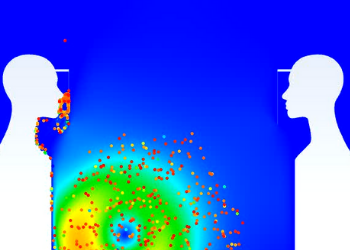
Team 22: Computational Flow Dynamic Model of COVID-19 Transmission and Face Shields
Eric Frank, Angela Lee, Brendan Reilly, Neel Sanghvi, Pranav Varrey
Advisor: Dr. Gregg Duncan (BIOE)
Face coverings, particularly face masks, have been vital in reducing the spread of COVID-191. While face masks have been heavily studied, less literature pertaining to face shield efficacy exists despite several benefits over face masks. Several advantages include easier disinfection, increased sustainability, comfort, and ease of communication within the deaf community. Per the National Deaf Center, face masks impair ASL communication by preventing lip reading and hiding facial features and expressions essential for speaking or signing communication, causing increased stress, fatigue, and anxiety. Underscoring that the deaf community has the right to communicate comfortably and safely in the midst of COVID-19, it is imperative to explore the efficacy of face shields in COVID-19 transmission prevention. With aerosol COVID particles being the main mode of transmission, computational fluid dynamic (CFD) models are pertinent to mathematically quantify and qualitatively observe particles interacting with the face shield and the wearer. Our 2D, transient state model utilizes literature-driven flow rates to simulate micron-sized particles being ejected from a mouth-like area under sneezing and breathing conditions. Simulations consisted of particle flow between permutations of two shielded and unshielded individuals. The model outputs each particle velocity and X, Y position that is then plotted to display the frequency of both velocity magnitude and particle distance travelled. The efficacy of the face shield is shown by comparing the particle distance travelled in the various simulations. With both individuals unshielded, the maximum distance traveled is 7.49ft with an average distance of 1.92 ± 1.95ft. Contrastly, with one individual shielded and the other unshielded, the maximum and average distance travelled decreases to 4.28ft and 1.20 ± 1.05ft respectively. Even with only one participant shielded, the model indicates that face shields significantly reduce particle transmission distances post-ejection into the surroundings by decreasing the frequency of droplets that travel farther distances (~6 ft). With the increased rate of vaccinations, the results imply that face shields could become more widely adopted to not only protect the wearer from COVID-19 but also permit the deaf community to comfortably and safely communicate and partake in society.

Team 23: Liquid Level Analysis through Machine Learning Imaging System Design
Ewuradjoa Amoah, Jonathan Kim, Natasha Kodgi, Adam Landa, Sarah Martin
Advisors: Dr. Huang Chiao (Joe) Huang (BIOE), Leon Tate (BD), Gaurav Falia (BD)
This project was sponsored by BD
Sepsis is a life-threatening condition that is often elusive of timely diagnosis and therefore requires faster processing and detection time of bacteria in blood samples for quicker diagnosis and treatment. To shorten the processing time, Team 23 team developed an imaging system component for automated medical diagnostic equipment. The component accurately identifies the liquid level of a patient blood sample that has been collected in a clear test bottle with a label and compiles barcode data scanned from the label with the liquid level analysis and stores the information in a unique patient file. It is highly effective (>96% accuracy) under different lightings and variable liquid levels conditions such as the presence of liquid foam. To ensure that their final design improves the accuracy and efficiency of the measurement of blood samples taken for sepsis detection, the group employed a Raspberry Pi 4 and HQ Camera Module in conjunction with a backend convolutional neural network (CNN) and real-time image analysis programmed in Python 3 utilizing OpenCV.
Published May 19, 2021









