News Story
Advancing Rapid Protein Analysis with Electronic Sensing

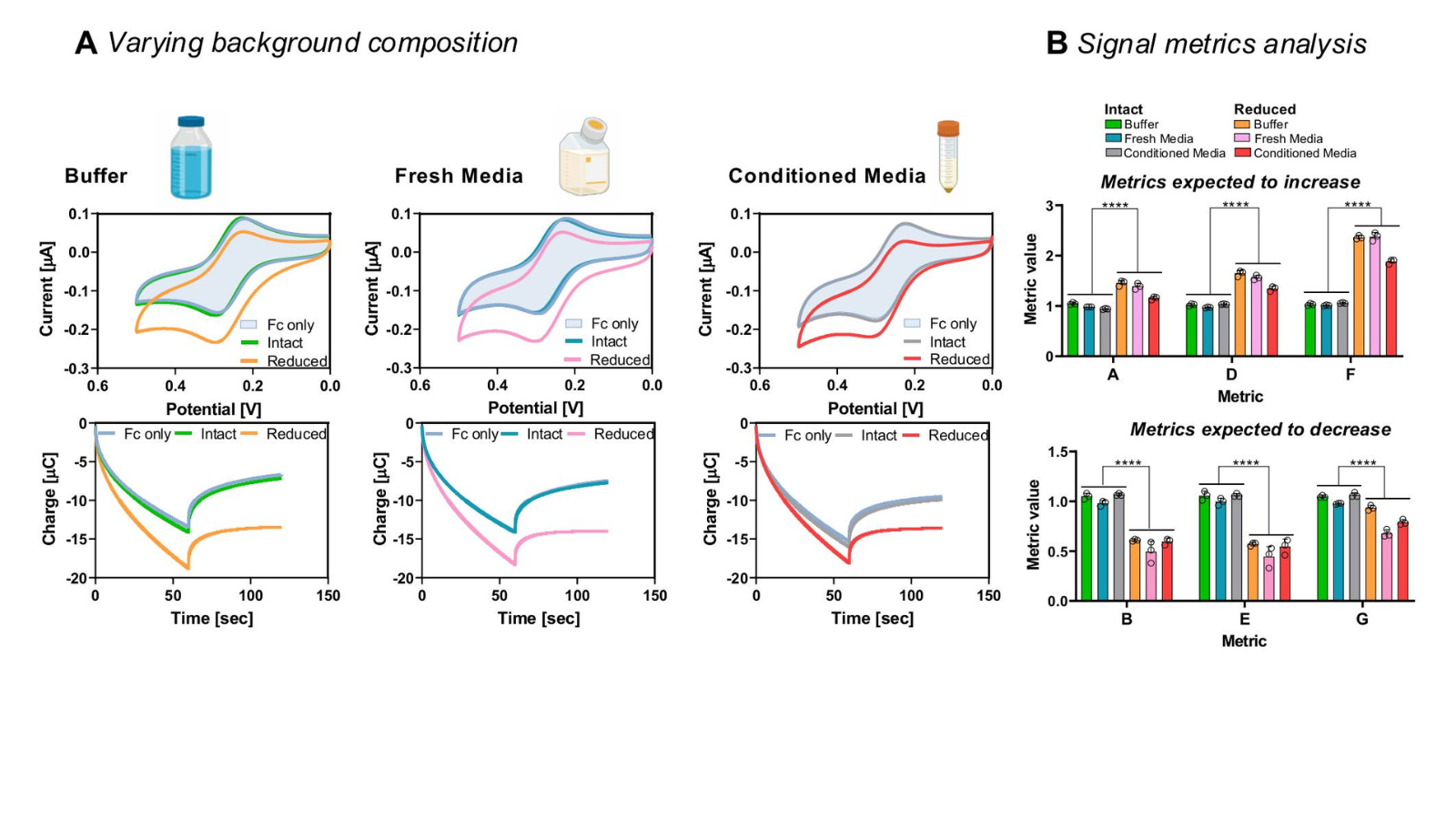
Biopharmaceuticals, such as monoclonal antibodies (mAbs), have revolutionized modern medicine, offering targeted treatments for diseases like cancer and autoimmune disorders. However, the structure of these complex proteins is critical to their effectiveness. During manufacturing and storage, subtle changes in their structure—such as oxidation or reduction—can compromise their function. To address this challenge, researchers at the University of Maryland (UMD), in collaboration with the Food and Drug Administration (FDA) and National Institute of Standards and Technology (NIST), have developed an innovative method to rapidly analyze protein structures using electronic sensing. This research was recently published in the peer-reviewed journal Nature Chemical Biology.
In their research, Robert E. Fischell Distinguished Professor and Director of the Fischell Institute for Biomedical Devices, William Bentley—alongside Fischell Institute Fellow and Institute for Bioscience and Biotechnology Research (IBBR) Professor Gregory Payne, BIOE alumna Dana Motabar (Ph.D. ‘24), and other researchers—used a technique called mediated electrochemical probing (MEP) to convert information about the proteins' structure into easy-to-read electronic signals. This method can identify common issues, like broken bonds or chemical changes within minutes.

“Imagine we might be able to rapidly and electronically assess how molecules drive biological function. Molecules are often indicators. Subtle changes in their structure might indicate their function. For example, an antibody that has disulfide bonds is more effective in eradicating a pathogen or a toxin. If we can detect these bonds (or their absence) we could have a better understanding of the robustness of our immune response.” Bentley explains. “If we can catch hold of this biological information in a simple electronic format, we can open entirely new pathways to improve health, food production, water quality, and even climate change by looking at electronic responses from an array of molecules.”
Traditional methods for analyzing molecules often require complex and costly tools, such as Nuclear Magnetic Resonance (NMR) machines or X-ray crystallography. In contrast, this innovative approach uses electrodes, potentiostats, and redox mediators—small molecules that transfer electrons—to rapidly and efficiently scan key molecular attributes. By translating biological information into an electronic format, the team’s work opens new possibilities for assessing molecules that influence health, food production, and the environment. This technology could simplify molecular analysis, making it faster, more affordable, and accessible beyond specialized laboratories.
“We think we have just scratched the surface. We are now incorporating genetically engineered cells into the bio-to-electronic information platform we've developed. We can now design cells to both detect specific molecules and produce electronic signals. This opens the door to creating new sensing technologies for a wide range of applications."
-Robert E. Fischell Distinguished Professor and Director of the Fischell Institute for Biomedical Devices, William Bentley

The method holds immediate promise for biomanufacturing, where ensuring protein quality is a major challenge. “There are few, if any, measurement techniques that allow for such a rapid and robust response.” Payne adds, “In the short term, the ability to read chemical information electronically will be useful in manufacturing as a means to monitor and control processes and product quality.”
The collaboration between UMD, FDA, and NIST has been central to this research's success. “NIST has developed an antibody reference material for researchers in industry and academia. We were incredibly fortunate to have access to this reference material. Because of this, researchers all over the world should be able to build off of our studies.” Bentley says.
Beyond simply detecting molecular changes, the team’s breakthrough lies in the rapid conversion of complex protein structure information into easy-to-measure electronic signals. This advancement has the potential to greatly improve the efficiency and reliability of biomanufacturing processes, ensuring the quality and safety of critical therapeutic proteins.

“We think we have just scratched the surface. We are now incorporating genetically engineered cells into the bio-to-electronic information platform we've developed. We can now design cells to both detect specific molecules and produce electronic signals. This opens the door to creating new sensing technologies for a wide range of applications."
Other contributors to this work include: (UMD) Eunkyoung Kim, Jinyang Li, and Zhiling Zhao (National Institute of Standards and Technology) Trina Mouchahoir, D. Gallagher, and John Schiel (US Food and Drug Administration) Mamatha Garige and Carole Sourbier.
Published January 24, 2025





