News Story
Stroka Wins NIH Grant for Cardiovascular Research
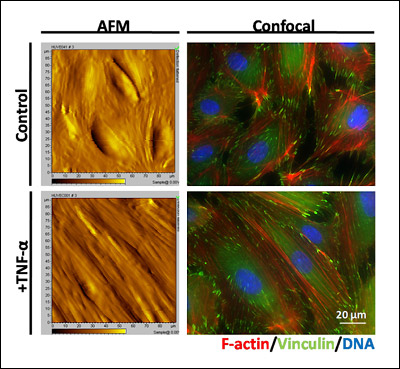
Above: This image shows both atomic force microscopy (AFM, left side) contact images and confocal images (right) of endothelial cell monolayers on a stiff surface, under both control and TNF-alpha-activated conditions. TNF-alpha is a treatment that induces the inflammatory response of endothelial cells and allows immune cells to attach to the surface. The AFM image allows Stroka and her colleagues to observe the topography of the endothelial cell monolayer, and at the same time take force measurements with the AFM to quantify the stiffness of the cells. The confocal image allows Stroka to observe the major sites of force transduction, i.e. the F-actin cytoskeletal network (red) and the focal adhesions (green). Cell nuclei are shown in blue.
Stroka, who conducts her research in the Cell Biophysics Lab, won the fellowship for her proposal to study how artery tissue stiffened by atherosclerosis (a type of cardiovascular disease, CVD) affects how leukocytes (white blood cells) enter through the endothelium, the layer of cells lining blood vessels. While her proposal is mainly targeted at understanding the forces involved as leukocytes migrate along and transmigrate through the endothelium, someday her results may help to find better ways to predict the onset of potentially deadly conditions such as strokes and heart attacks.
The role of leukocytes in CVD is central in Stroka's study. Leukocytes are the soldiers of the human immune system that are called to respond to injury and fight disease. But in an ironic twist, she says, their behavior may lead to CVD under certain conditions.
When leukocytes transmigrate into a blood vessel where endothelial cell walls have been damaged by low-density lipoproteins (LDL, popularly known as "bad cholesterol"), their response is to remove the problem by absorbing the LDL. "Unfortunately, the leukocytes aren't capable of breaking down the LDL, and this eventually leads to a pathological state," Stroka explains. "The leukocytes turn into what are called 'foam cells' and form a plaque beneath the endothelial cells. If this plaque continues to grow, it eventually becomes so large that it obstructs blood flow, which could lead to a stroke or a heart attack. So atherosclerosis is actually caused by an increase in the activity of the cells that are supposed to protect us."
Stroka will be studying the mechanical stresses that affect the damaged endothelial cells during the process: shear stress from the flow of blood above them and the stress exerted by the stiffening of the arterial tissue on which they rest. To accomplish this, she has devised a new, more accurate way of simulating healthy and diseased arteries in the lab that will allow her to both control and apply different combinations of forces to cultured endothelial cells. Using methods such as atomic force microscopy, traction force microscopy, and confocal microscopy, she plans to measure how these combinations of forces affect both the mechanical properties of the endothelial cells and also the process of leukocyte transmigration. The results could provide a clearer and more detailed explanation of the conditions leading to and progression of atherosclerosis.
"In the future," says Stroka, "we might be able to gauge the onset of atherosclerosis or an oncoming stroke based on the behavior of a patient's immune system, or develop new drugs to restore elasticity to the arteries. In order to accomplish that, we need a quantitative understanding of leukocyte transmigration and the forces involved. It's going to be crucial to the development of better diagnostics and treatments for cardiovascular disease."
For More Information, See:
Published January 19, 2010










