News Story
Python-Based Program Could Advance Understanding of Blood-Brain Barrier

Dr. Kimberly Stroka
University of Maryland Fischell Department of Bioengineering (BIOE) researchers have developed a novel analyzer program to advance understanding of the body’s blood-brain barrier and the role it plays in neurodegenerative disease.
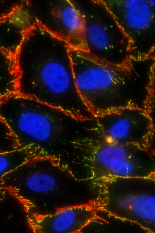
The blood-brain barrier is a highly selective semi-permeable barrier made up of endothelial cells that line the blood vessels in the brain. This barrier plays a critical role in protecting the brain from foreign substances in the blood, while allowing essential nutrients to reach the brain.
This selective permeability of the brain endothelial cells is controlled by what are known as tight junction proteins. Tight junctions serve as fusion points between cells, and are made up of numerous proteins that join together to form a barrier to fluid.
Put simply, the blood-brain barrier wouldn’t function without tight junction proteins.
In fact, disrupted junctions are linked to several neurodegenerative diseases such as Alzheimer’s disease and multiple sclerosis – but, scientists don’t yet fully know what causes these disruptions. Understanding the key players driving junction stability could therefore hold significant promise for therapeutic discovery. On the other hand, understanding how cell-cell junctions operate in brain endothelial cells could improve drug delivery into the brain, which is often limited by an intact blood-brain barrier.
Knowing this, BIOE Assistant Professor Kimberly Stroka (Ph.D. ’11) led a team of researchers in her Cell and Microenvironment Engineering Lab in developing the Junction Analyzer Program (JAnaP), a novel, Python-based program capable of providing quantitative metrics that describe the integrity of cell junction proteins in response to different biochemical and physical cues. Such a program could prove invaluable as, to date, there are no models or techniques that can be used to efficiently quantify junctional proteins as they are assembled at the cell edge.
 The work put forth by Stroka and her team is published in the July 2019 edition of Annals of Biomedical Engineering. BIOE Ph.D. student Kelsey Gray, a member of the Stroka Lab, is lead author on the paper.
The work put forth by Stroka and her team is published in the July 2019 edition of Annals of Biomedical Engineering. BIOE Ph.D. student Kelsey Gray, a member of the Stroka Lab, is lead author on the paper.
“The standard in vitro methods for assessing endothelial cell permeability typically only provide global measurements of electrical resistance or permeability to fluorescent molecules. That means that you would get one number that you would have to assume is representative of the whole sample,” Stroka said. “The standard methods don’t provide any kind of quantitative assessment of localized alterations in cell-cell junction integrity, which would be important in more complex environments where there are spatial and temporal heterogeneities in cell behavior. As we work toward building better in vitro vascular models with appropriate mechanical and biochemical cues integrated within, it’s critical that we also have efficient tools, such as the JAnaP, to provide quantitative assessment of cell functionality.”
Previous studies have linked edge-presentation of what are known as vascular endothelial cadherins (VE-cadherins) with junction and barrier maturity. VE-cadherins are a type of transmembrane protein critical to tissue structure. When the extracellular segments of these proteins bind to one another, they help form cell-cell complexes called adherens junctions, which provide structural support between adjacent cells. In the case of the blood-brain barrier, adherens junctions work together with tight junctions to stabilize brain microvascular endothelial cells and maintain normal physiological processes.
Earlier findings have indicated that how VE-cadherins assemble at the cell edge can impact both junction and barrier stability and maturity. As such, Stroka and her team believe the JAnaP could help advance understanding of the conditions that influence specific junction presentation and how these junctions contribute to certain blood-brain barrier properties. Armed with this knowledge, researchers would be better positioned to develop therapeutics capable of traversing the blood-brain barrier for delivery to the brain or for diseases associated with blood-brain barrier dysfunction.
“We are now working on determining how specific tight and adherens junction morphologies – and their dynamic rearrangement – contribute to local permeability in different regions of the endothelial cell monolayer,” Stroka said. “We are also working with collaborators interested in using information from the JAnaP to improve drug delivery across the blood-brain barrier. We are optimistic that the JAnaP can be useful in both fundamental and applied research involving vascular barriers.”
In addition to Stroka and Gray, former BIOE undergraduate students Dakota Katz (B.S. ’16) and Erica Brown (B.S. ’18) served as co-authors of the Annals of Biomedical Engineering paper titled, “Quantitative Phenotyping of Cell–Cell Junctions to Evaluate ZO-1 Presentation in Brain Endothelial Cells.” Katz is a current biomedical engineering Ph.D. student at Washington University, and Brown currently works with MedImmune.
Published June 25, 2019